A solution at room tempearature with a pH of less than 7 will be:
As temperature increases, the pH of a sample of pure water will:
What is the value of Kw for a sample of pure water at room temperature?
What is the hydroxide ion concentration of a solution at 25∘C with a pH=9.90?
Which substance has a pH that is higher than pure water?
Water’s pH and pOH are both 7 at:
A solution at room tempearature with a pH of 7 will be:
Which of the following are acidic at room temperature?
What is the pH of a solution with [H3O+]=1.50×10−6 M?
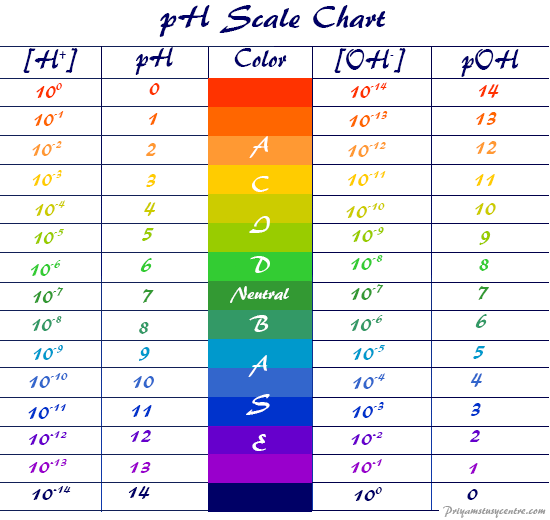
pH Scale – pOH Scale – Definition, Range, Chart, Measurement
What is the hydronium ion concentration in a solution of pH 10.90?
Calculate the pOH of a solution that has [H3O+]=0.050 M at 25∘C.
What is the hydroxide ion concentration of a solution at 25∘C with a pH of 7.40?
Which value for pOH corresponds to an acidic solution at room temperature?
If coffee has a [H3O+] of 10−5 M and an [OH−] of 10−9 M, it is:
The solution of aluminum hydroxide
What is the concentration of hydronium in a sample of pure water at room temperature?
The lowest value that is possible on the pH scale is:



